Quality Event Management is crucial in the Life Sciences for ensuring compliance with regulations, enhancing patient safety, and maintaining product quality. It can also lower costs, reduce development errors, and enable a shorter time to market.
Avertim's consultants have extensive experience supporting pharmaceutical and medical device businesses in this area.
HERE'S OUR APPROACH.
Quality Event Management in the Life Sciences: An Overview
Deviation Management is the systematic recording, investigation, and evaluation of deviations. The goal is to determine the cause of the deviation, evaluate its influence on product quality and safety, and prevent it in the future through Corrective and Preventive Actions (CAPA).
Change management ensures that actions leading to controlled and coordinated changes in processes, systems and documents are thoroughly planned, evaluated, approved and documented. It is crucial, not only for activities deriving from CAPAs but for all GMP-relevant (Good Manufacturing Practice) alterations, such as the introduction of new raw materials, supplier transitions or the adoption of new analytical methods.
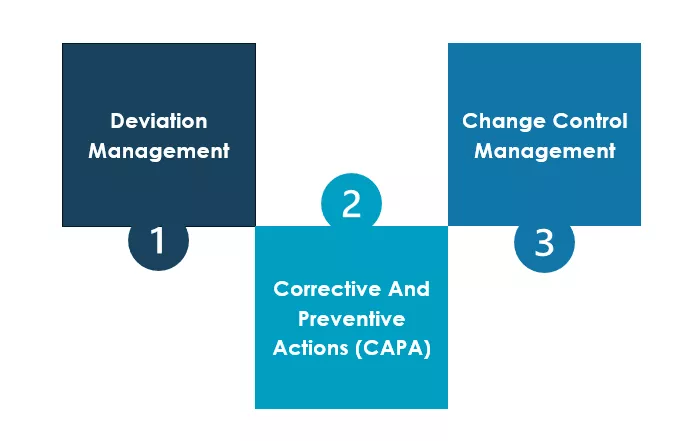
QEM is essential for pharmaceutical and medical device manufacturers to ensure consistent product quality and patient safety, as well as compliance to product and process related requirements imposed by legislation and regulations.
Ensuring quality and safety - the industry challenge
The pharmaceutical and medical device industries have many challenges, including those related to regulation, processes, and training.
Regulations:
From a regulatory perspective, keeping up with diverse and changing requirements across agencies is a persistent challenge, as well as the increased workloads before and after authority inspections and audits.
Processes:
Inadequate compliance and management of the quality systems (such as deviations, CAPA and changes), as well as deficient digitalisation, are typical process-related challenges.
Trainings:
Training-related challenges involve inadequate training programs, irregular development initiatives for new technologies and techniques and insufficient certification of employees.
Effectively addressing these challenges and promoting industry competitiveness requires the implementation of "Good Practice", or GxP-compliant QEM and associated processes. This involves :
- Establishing compliant, efficient, and standardised processes
- Optimising cross-departemental communication and coordination
- Increasing use of digitalisation and automation
- Coherent training coordination and control
Avertim's approach to Quality Event Management
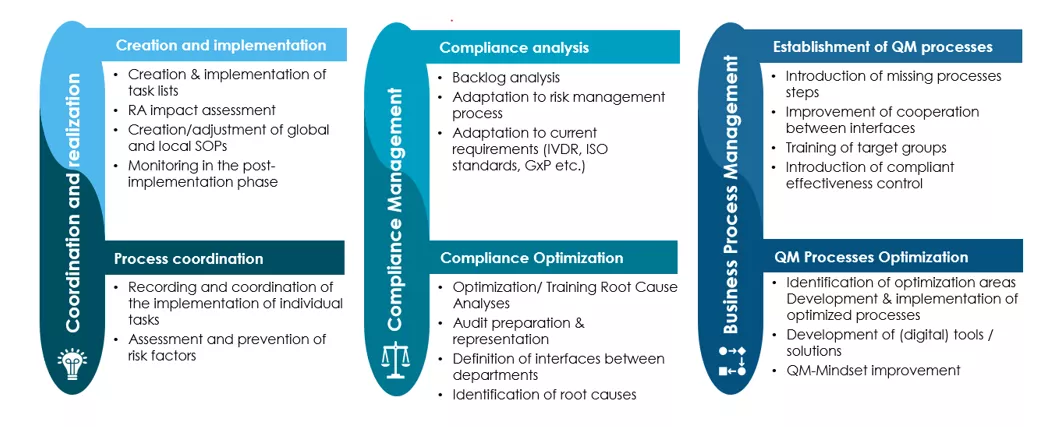
Avertim's experienced consultants support your company in the three core areas of QEM:
- Coordination and realisation
- Operative support through application of best-practice methods for end-to-end quality systems management.
- Compliance Management
- Proactively managing regulatory risks and conducting of analyses for an adaptive compliance approach, ensuring readiness for audits.
- Business Process Management
- Continuous improvement of process and business-related strategies in line with industry 4.0 principles.
Realising the benefits of Quality Event Management (QEM) with Avertim
QEM integrates systematic improvement into the process structure,while complying with legal and contractual regulations. Our expertise enables the significant reduction of entreprise-wide risks, as well as planning and evaluation of necessary optimisation measures.
In Avertim's consulting experience, this generally results in :
- Lower cost minimised risk quality processes
- A reducation of errors in the development process
- Improved interdepartmental Quality Management coordination
- Accelerated project/measure planning and implementation
- And reduce time to market entrance
Process structures become more robust, more effective and more efficient. This leads to economic and qualitative progress, and increased competitive advantage.
Avertim's skilled professionals are here to support your business. We can provide the specific Quality Event Management experience you need to ensure quality, compliance and safety, and to unleash your potential for improvement.


