A thorough approach to incoming materials management ensure the physical health of patients - and the financial health of business. Avertim have been supporting the pharmaceutical industry in complying and excelling since 2007.
Here's what we know.
Incoming Materials Management in the Life Sciences - an overview
Supply chain activities are key to ensuring medicines can be manufactured and delivered to patients on time. Weak or unstable supply plans or insufficiently controlled supply chains can lead to serious business continuity concerns or even put the product's quality and safety at risk.
Deliberately, or unknowingly, suppliers to the pharmaceutical industry may take less expensive routes of manufacturing, packaging or transportation. This has potential to directly impact the supplied material's quality through unexpected and unwanted contaminations. The financial and legal costs of investigation and reruns can be extremely high.
Thorough incoming material selection (eg. chemical and biological raw materials, single-use-systems, excipients, etc.) and appropriate qualification activities along the supply chain ensure drug products have a strong foundation. This means greater safety for both the physical health of patients and the financial health of business.
Ensuring efficiency and safety - the industry challenge
The pharmaceutical and medical device industries face simultaneous regulatory and optimisation challenges.
1. The qualification, supply and release of Incoming Materials is in constant focus as players in the pharmaceutical industry aim to optimise production efficiency and costs.New technologies and ways of working - such as Single Use System (SUS), reduction of release testing or the externalisation of qualification activities - are important in business process optimisation.
2. At the same time, regulatory requirements are always increasing. Pillars of compliance management include transparent supply chains, certified packaging materials, and detailed information of potential contaminents.
3. Incoming materials suppliers are urging for optimisation measures as well. Costly issues include: the efficient control of international supply chains; customer support for qualification; and maintenance of relevant certifications.
4. Increasing customer requirement add to the challenge. Extensive qualification questionnaires and quality agreements, regular auditing along the supply chain, and disclosure of traditionally confidential information, are all issues faced.
Avertim's approach to Incoming Materials Management
Avertim have extensive expertise; we have been supporting the pharmaceutical industry in their compliance and business process management since 2007.
Quality Gap analyses, alignement against regulatory or corporate requirement, and gap categorisation, lay the grounds for optimisation measures.
Risk Based prioritisation, definition and implementation of CAPAs and changes, creation of interfaces between departments, and identification of sources of errors, take clients to the next level with individual optimisation strategies.
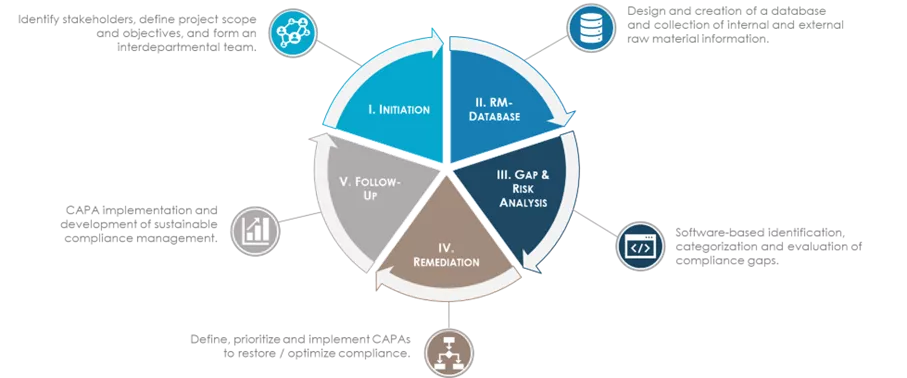
Performance analyses, control strategies, identification of optimisation potentials, and development of digital tools are amongst the new business standards for optimising qualification processes.
A robust end-to-end qualification process that covers the materials' entire life cycle is a crucial foundation to the management of materials and suppliers. Ensuring the awareness of all process stakeholders through adequate training is essential.
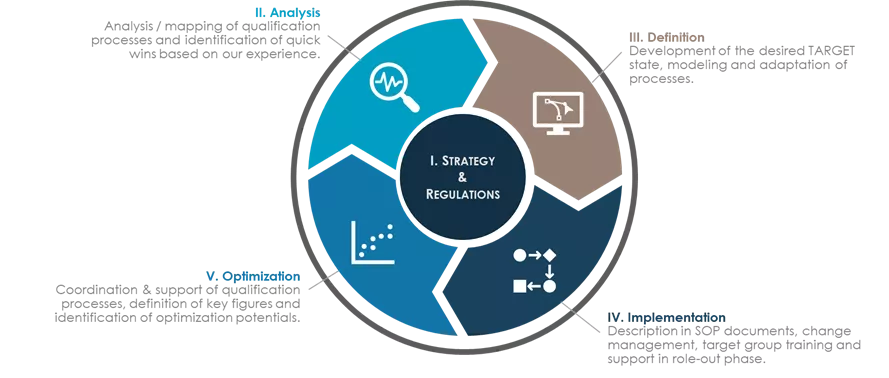
Realising the benefits of IM Management with Avertim
Avertim has effectively supported the qualification of hundreds of new incoming materials (chemical, biological, and single-use-systems) and their suppliers in the pharmaceutcal industry.
Support ranged from the planning and coordination of qualification activities to specific qualification actions. These include reviews of materials' audit and QAA statuts, preparation of compliance assessments, and creating guidance documents and jobs aids.
Translating the client's strategy into actionable projects, we facilitate risk analyses and mitigation with supply chains' analysis as well as quality gap assessments. This includes perspectives on the given supply chain complexity, raw material quality, audit history, GMP and pharmacopoeia compliance. There is also the development of risk mitigation strategies and conducting workshop series to enable implementation.
Using bespoke solutions, Avertim has a successful track record of establishing holistic incoming material control strategies, ensuring internal corporate as well as external regulatory requirements.
Our extensive experience allows us to advise and support means of optimisation, for example through software tools based on the customised qualification process as well as the control strategy. This results in an increased robustness approach to incoming material management at client sites.
We ensure the integration of optimisation measures into the corporate framework to unleash the full improvement potential, such as consolidating the site-wide SOP landscape and trainings for qualification experts after successfully establishing a new end-to-end supplier and materials qualification process.
Avertim makes a real difference on the incoming material compliance of clients. Our skilled and experienced professionals are here to support you in your compliance and in the development of individual optimisation strategies. With our range of specialised methodologies and techniques, we can provide the specific experience in material and supplier qualification you need to both comply and excel.


